For home administration of ORALAIR, your doctor should prescribe auto-injectable epinephrine for you to keep at home for treating a severe reaction, should one occur. Your doctor will train and instruct you on the proper use of auto-injectable epinephrine.
ORALAIR can cause severe allergic reactions that may be life-threatening. Symptoms of allergic reactions to ORALAIR include:
- Trouble breathing
- Throat tightness or swelling
- Trouble swallowing or speaking
- Dizziness or fainting
- Rapid or weak heartbeat
- Severe stomach cramps or pain, vomiting, or diarrhea
- Severe flushing or itching of the skin
If any of these symptoms occur, stop taking ORALAIR and immediately seek medical care.
Please see additional Important Safety Information below and full Prescribing Information, including Boxed Warning and Medication Guide.
How to take ORALAIR

Remove the ORALAIR tablet from the blister pack right before you take it.
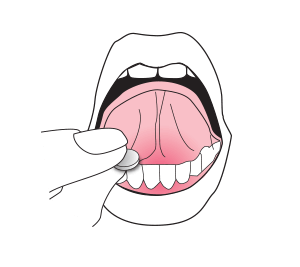
Place the ORALAIR tablet under your tongue right away. Keep the tablet there for at least 1 minute, then swallow.

Take the ORALAIR tablet without food or drink. Do not eat or drink anything for 5 minutes after you take ORALAIR.

Be sure to wash your hands after handling the ORALAIR tablet.
ORALAIR Dosing
Once ORALAIR is started in the allergy specialist’s office about 4 months before the grass allergy season begins, adult patients 18 to 65 years old will take 1 ORALAIR 300-IR tablet daily until the end of the grass allergy season. Children and adolescents 5 to 17 years old will take 1 ORALAIR 100-IR tablet in their allergy specialist’s office on the first day of treatment about 4 months before the grass allergy season begins; after that, they will take 2 ORALAIR 100-IR tablets on their second day of treatment, and then 1 ORALAIR 300-IR tablet on the third day and every day moving forward, until the end of the grass allergy season. ORALAIR should always be given to children under adult supervision.
At-home dosing for ORALAIR
treatment as prescribed
1 ORALAIR 300-IR
tablet each day

2 ORALAIR
100-IR tablets

1 ORALAIR 300-IR
tablet each day




Your allergy specialist will tell you when to stop taking ORALAIR.
If you forget to take ORALAIR, do not take a double dose. Take the next dose at your normal scheduled time the next day. If you don’t take ORALAIR for more than 1 day, contact your allergy specialist before restarting.
Remembering to
Take ORALAIR
If you aren’t yet experiencing allergy symptoms, remembering to take ORALAIR every day can be difficult. Here are some helpful tips:
Take ORALAIR at the same time you do another daily activity that you never forget, like getting dressed in the morning or feeding a pet
Set a reminder alarm on your cell phone or computer, or put a sticky note where you will always see it, like on your bathroom mirror or refrigerator
Keep your ORALAIR tablets in the same place where you can easily get to them
Use the ORALAIR Treatment Tracker to record your allergy medicines and grass allergy symptoms every day

